- STORIES
MyEni Login

Magnetic confinement fusion: instruction for use is an original format by Eni that aims to explain various aspects related to a technology with enormous potential.
As we know, everything around us, including ourselves, is made up of molecules. Molecules are made up of atoms bonded together. And atoms, in turn, are made up of protons and neutrons - which are in the nuclei - and electrons - which move around the nuclei.
The number of protons in the nucleus of an atom tells us which element we are dealing with. Hydrogen has only one proton, Helium two, Lithium three and so on until Uranium with ninety-two. In the Periodic Table, there are actually a handful of other elements with more than ninety-two protons, but they don’t exist in nature. They have to be built in large particle accelerators and almost all of them survive for less than an instant. For now ... we can leave them to one side.
What are isotopes?
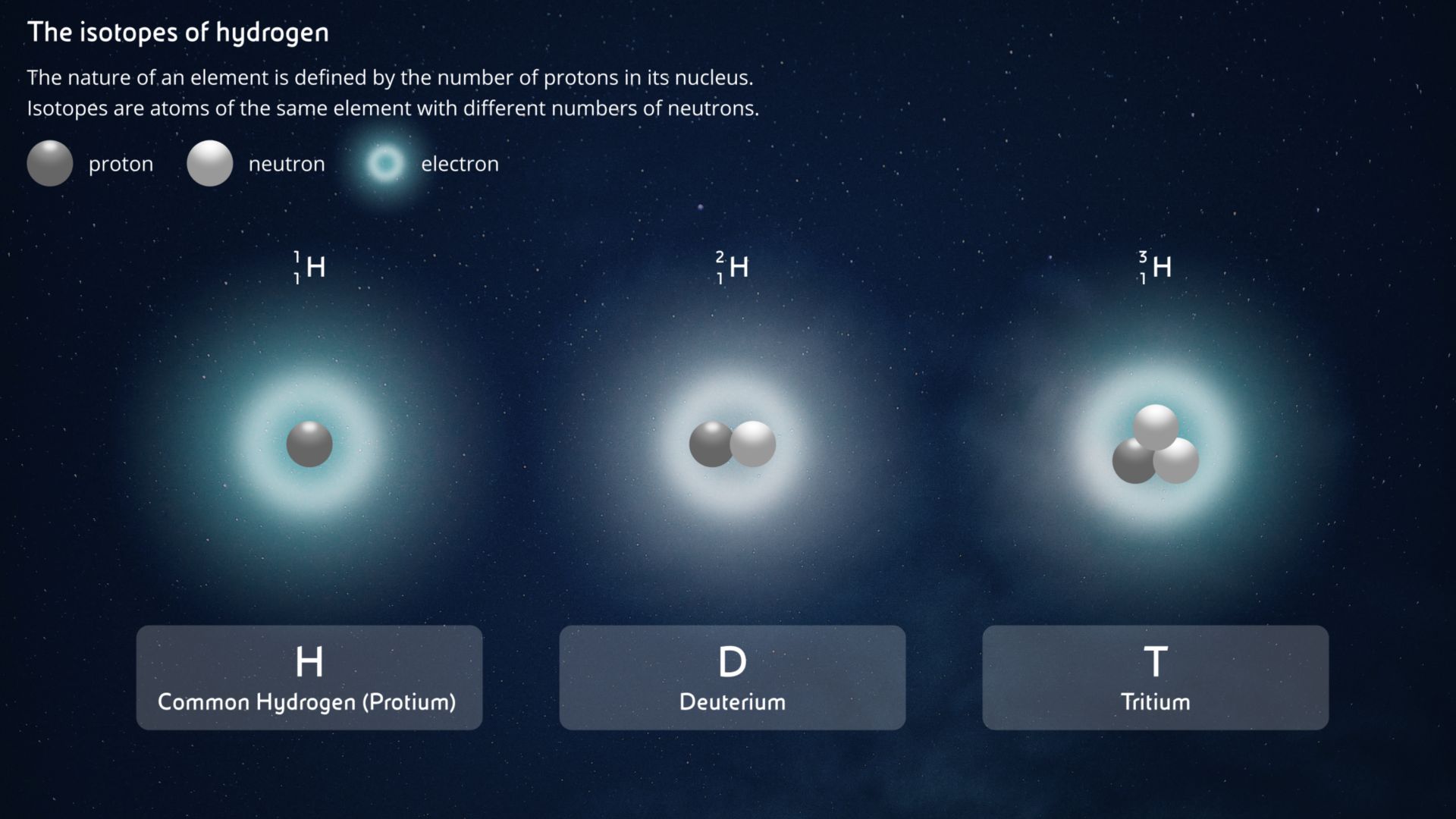
Inside the nucleus there are also neutrons, which - as their name suggests - have no electrical charge, unlike positively charged protons. If two nuclei with the same number of protons (i.e. of the same element) have different numbers of neutrons, they are called isotopes. The number of neutrons in a nucleus tells us exactly which isotope we are talking about. Chemically, the isotopes of a certain atom are all the same because they have the same number of protons and electrons, which determine their chemical properties. Only the mass of their nucleus changes (a neutron weighs just a little more than a proton, and the sum of protons and neutrons gives us the total mass of the nucleus).
The nucleus of the most common isotope of Hydrogen has only one proton and no neutrons. Everyone calls it simply Hydrogen, but its real name is Protium. When there is also a neutron in a Hydrogen nucleus, we have Deuterium. In nature, for every 6670 atoms of Protium, there is only one Deuterium atom. And when we have two in the Hydrogen nucleus we have Tritium, which is unstable and only exists in traces, but can be produced artificially.
Hydrogen is the only atom to have isotopes with their own names. All isotopes of other atoms are distinguished only by the numbers written in the upper left-hand corner of their atomic symbol. For example, the most stable isotope of uranium is 238U. This is a uranium atom (92 protons) with a total mass of 238, i.e. also containing 238-92=146 neutrons. On the other hand, 235U still has its 92 protons (otherwise it wouldn't be uranium!) but "only" 235-92=143 neutrons.
This is almost all we need to know about how nuclei are made up, unless we are seriously considering a degree in nuclear physics. Now all that remains is to understand what forces are at work inside these nuclei. While we're at it, we'll also talk about all the forces that are at work in the Universe. Don't panic, there are only four!
As we said, as far as we know today, all the forces of the Universe can be attributed to four fundamental forces.
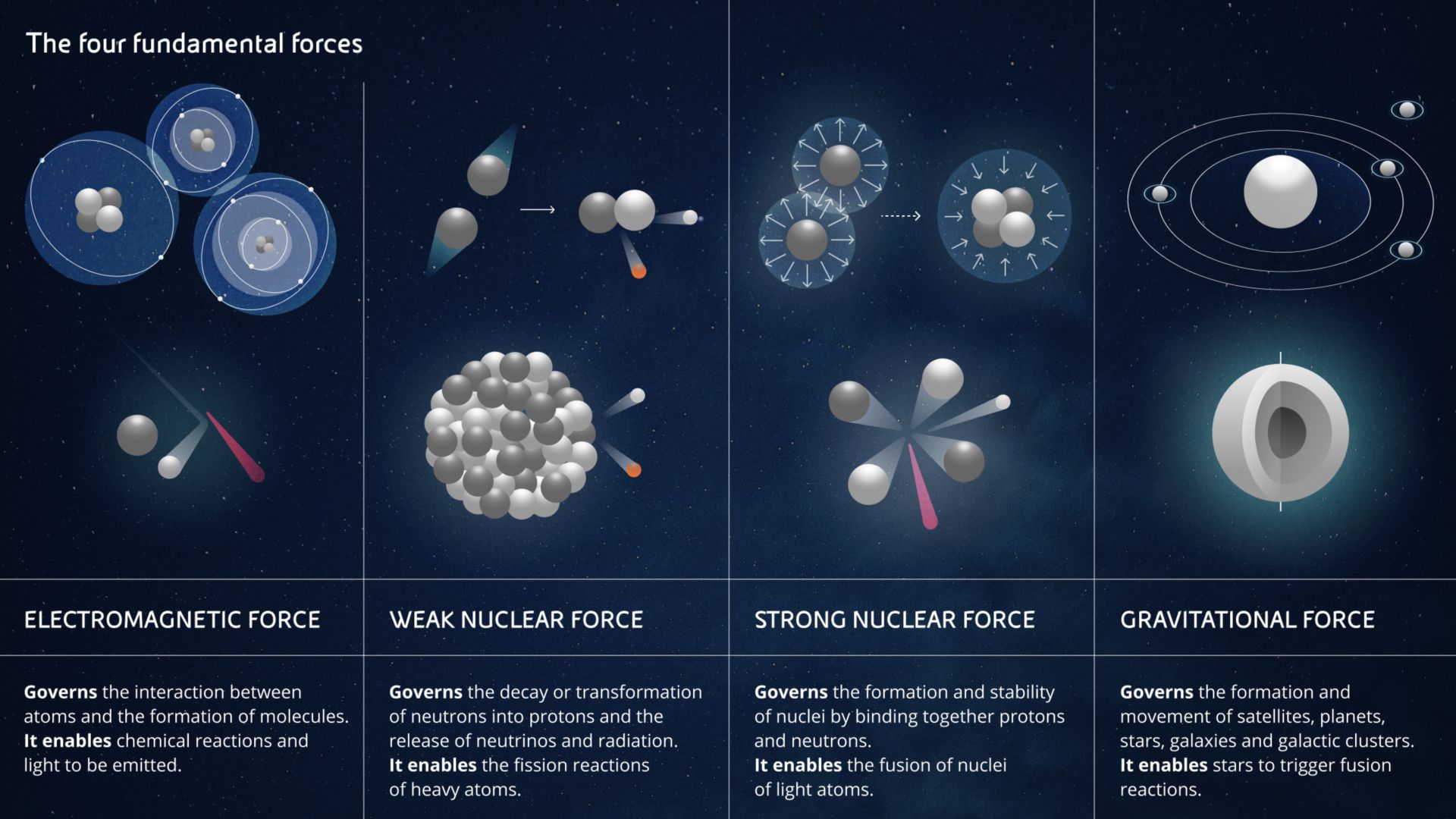
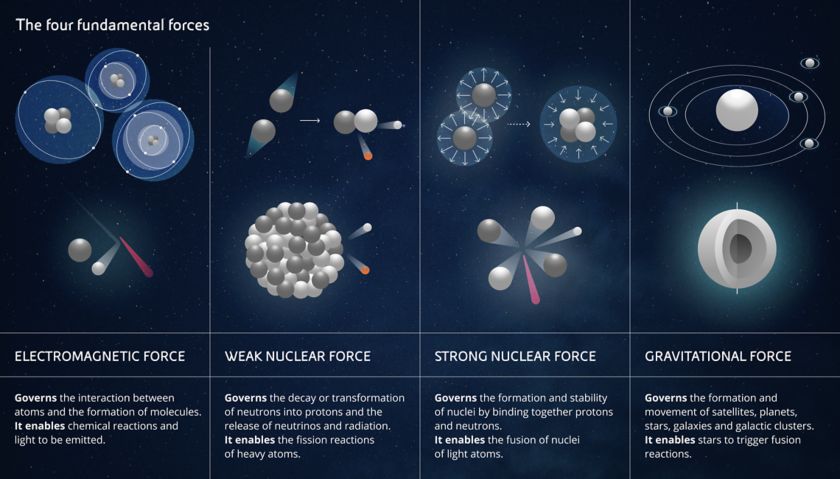
- The electromagnetic force is the force that allows you to read these words thanks to the electricity powering your device, but it is also the force that produces photons, i.e. light. At a microscopic level, the electromagnetic force acts between individual charged particles. But when many charged particles are working together, you can make much bigger things work, like electric motors, and you can perform chemical reactions. You might never have thought about it, but even when you kick a ball, the electrons on the outer surface of your shoe repel those on the surface of the ball. So even a football match is a scientific experiment.
- The gravitational force acts on all bodies with mass. It is the weakest of all the forces but is evident even at a great distance. It is the force that made the apple fall on Newton's head, the one that holds the Solar System and the Galaxies together, but it is also the force that creates enormous pressure within stars. This pressure allows the stars themselves to trigger and sustain hydrogen fusion and makes them glow for billions of years.
The final two forces are a little less visible than the others, but just as fundamental:
- Weak nuclear force and strong nuclear force. From their names, it is immediately clear that they act in the nucleus of atoms. In particular, the former is responsible for converting neutrons into protons and electrons, as well as for radioactivity, while the latter serves to keep the protons and neutrons of a nucleus bound together and allow atoms to... exist. It is this which, by counteracting the electrical repulsion between positive particles, prevents each proton in a nucleus shooting away from all its other companion protons.
When you manage to overcome the strong nuclear force between protons and neutrons of heavy atoms - physicists define atoms as heavy when they have more protons than iron, which has 26 - you can release a lot of energy. Even more energy can be released if light atoms - i.e. those with fewer protons than iron - are fused together, which we will talk about later when we discuss nuclear fission and fusion.
The author: Luca Longo
Industrial chemist specialized in theoretical chemistry. He was a researcher for 30 years before moving on to Eni's scientific communication.
Magnetic confinement fusion
All the news and insights in original texts, videos and podcasts on the technical challenges and industrial developments of a revolutionary technology.

Eni.com is a digitally designed platform that offers an immediate overview of Eni's activities. It addresses everyone, recounting in a transparent and accessible way the values, commitment and perspectives of a global technology company for the energy transition.
Discover our mission